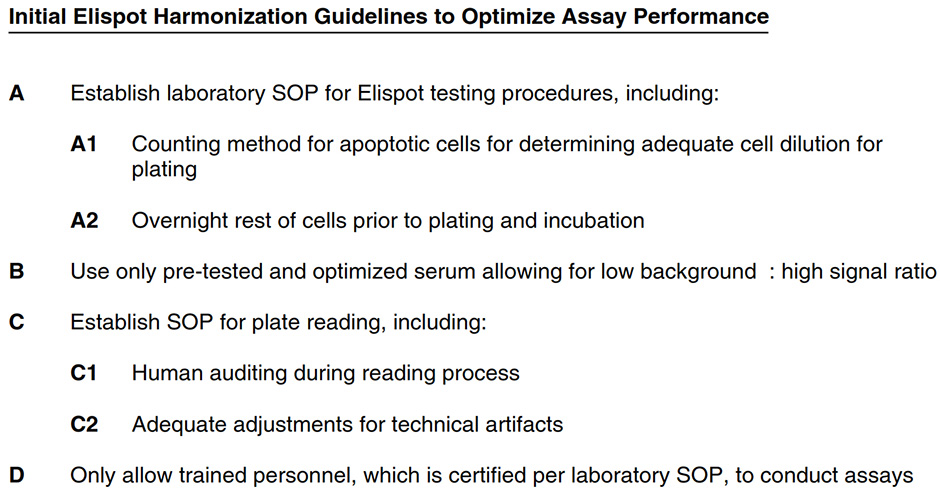
The following initial Elispot harmonization guidelines have been established in large-scale proficiency panels conducted by the Cancer Immunotherapy Consortium (CIC). They summarize critical protocol steps which influence the assay outcome. Integration of these guidelines is recommended to achieve optimal assay performance. Each laboratory needs to check the influence of newly implemented guidelines on final assay outcome before generalized use of the adapted SOP. Harmonization guidelines can be implemented independent of used reagents, materials and equipment. Harmonization does not require strict standardization based on “one” SOP.
Resources:
- Original Elispot Harmonization paper from CIC efforts:
Click to read online - Elispot Plate Reading Guidelines – International Effort
- Publication about serum-supplemented and serum-free media for IFNɣ Elispot:
Click to read online - The effect of harmonization on Elispot performance
Click to follow Link - Assay harmonization paper – 2008 -Quo vadis?
Click to read PDF - Publication in Science Translational Medicine about harmonization of biomarker assays:
Click to follow Link
